Quantum Tunnnelling in Enzymes
Enzymes are the workhorses of life. They speed chemical reactions so that processes that would otherwise take thousands of years proceed in seconds inside living cells. But how they accelerate chemical reactions by such enormous factors, often more than a trillion-fold, has remained an enigma. Recent research, particularly by Judith Klinman at the University of California and Nigel Scrutton at the University of Manchester, has shown that several enzymes use another weird quantum trick called tunnelling to accelerate biochemical reactions. Essentially, the enzyme promotes a process whereby protons vanish from one position in a biochemical and instantly rematerialize in another, without visiting any of the in-between places – a kind of quantum teleportation. Enzymes have made every single biomolecule in your cell and every cell of every living creature on the planet so they are, more than any other component (even DNA, as some cells get by without it) the essential ingredient of life. And they dip into the quantum world to help keep us alive.
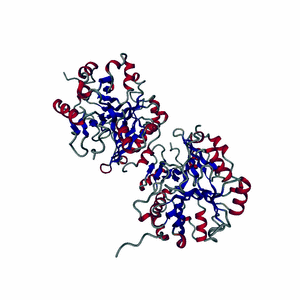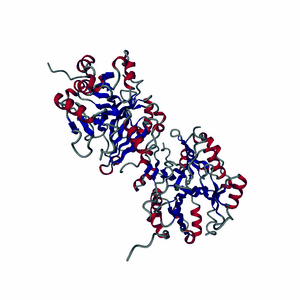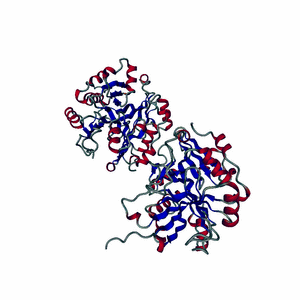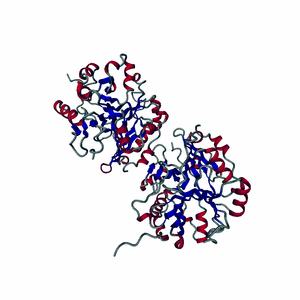
Enzymes are made of highly-structured ribbons of amino acids that are held together by ionic bonds to form complex three-dimensional shapes that are capable of twisting and bending around a substrate.
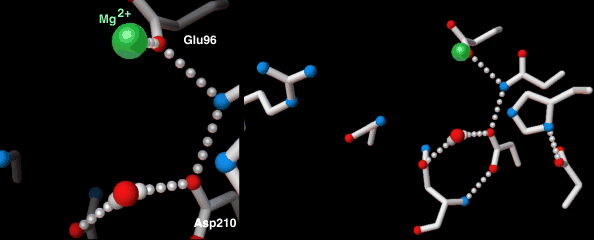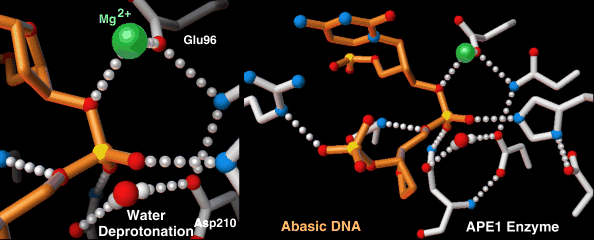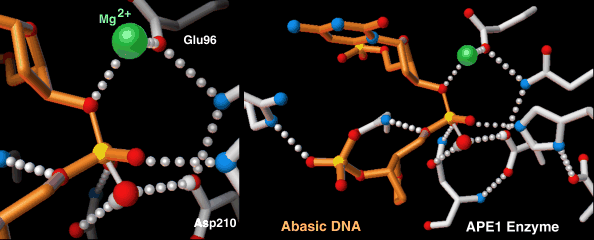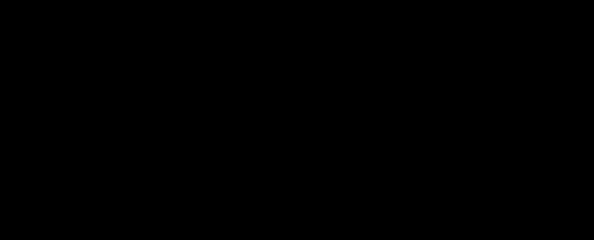
Highly-choreographed molecular motions of atoms and fundamental particles take place within the active site of an enzyme.
In quantum mechanics, particles don’t have defined positions in space but their position is instead defined by a diffuse wave function. This positional fuzziness (an aspect of Heisenberg’s famous uncertainty principle) allows the edges of particle waves to leak through classical barriers, a process known as quantum tunnelling. Tunnelling is involved in phenomena such as nuclear decay and also responsible for the sun shining (and thereby life on Earth). Considered classically, solar protons (hydrogen nuclei) have insufficient energy to penetrate their mutual electric repulsion barrier in order to fuse and generate a deuterium nucleus (the first step in the fusion process that powers the sun. However, in quantum mechanics, the proton’s positional fuzziness, its wave function, can leak through the barrier via quantum tunnelling to initiate the nuclear fusion reaction.